A pharmacist prepares to administer COVID-19 vaccine booster shots at a senior center in September 2022 in Chicago, Illinois. The recently authorized booster vaccine protects against the original SARS-CoV-2 virus and the more recent omicron variants, BA.4 and BA.5. (Photo by Scott Olson/Getty Images)
BY AND
On August 31, 2022, the U.S. Food and Drug Administration (FDA) amended the emergency use authorizations (EUAs) of the Moderna COVID-19 vaccine and the Pfizer-BioNTech COVID-19 vaccine to authorize the use of bivalent COVID-19 vaccines manufactured by both the companies as a single booster dose.
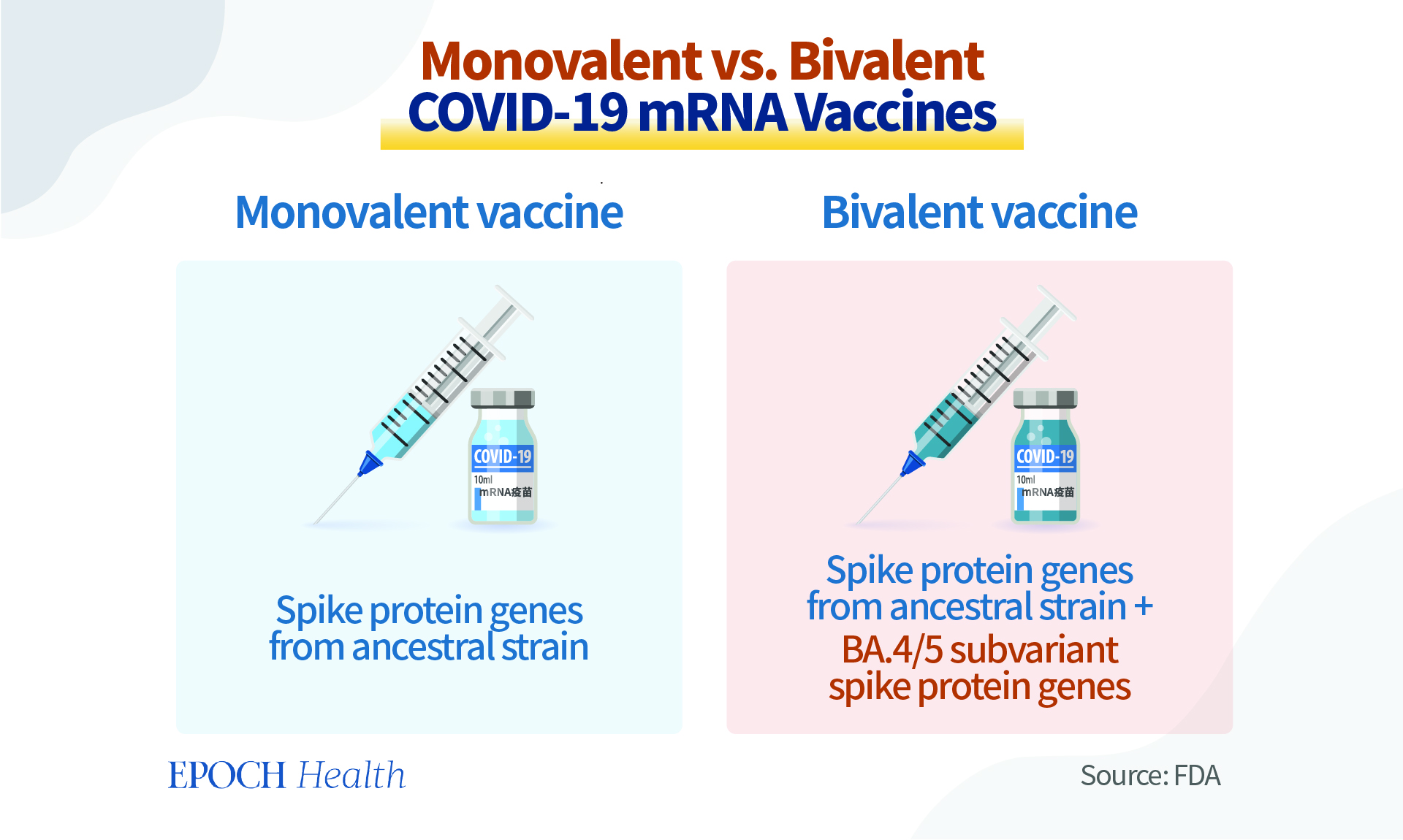
These bivalent vaccines contain the mRNA components of both the spike genes of SARS-CoV-2 virus ancestral strain and the BA.4/5 subvariants. However, the ancestral strain has long disappeared. So why should the new booster vaccines still incorporate half of the original strain’s mRNA, when the Omicron variants are currently the most prevalent?
What Are These Updated Boosters?
These bivalent vaccines are called “updated boosters” by the FDA. They contain two messenger RNA (mRNA) components of the SARS-CoV-2 virus, which is the culprit of the COVID-19 pandemic. One component is the ancestral strain of SARS-CoV-2, which was isolated and sequenced from Wuhan, China; and the other is the mRNA shared by the BA.4 and BA.5 sub-lineages of the Omicron variant.
The mRNA in these vaccines is a specific piece of genetic material that instructs cells in the body to make the SARS-CoV-2 spike protein that will trigger an immune response inside the body.
These new bivalent vaccines have been designed to mainly combat the BA.4 and BA.5 subvariants, as they are currently the most prevalent strains in the United States, as per the Centers for Disease Control and Prevention (CDC). These subvariants are also predicted to circulate in the fall and winter of 2022. In June 2022, the FDA’s Vaccines and Related Biological Products Advisory Committee voted to include an Omicron component in the new COVID-19 booster vaccines.
The previous COVID-19 mRNA vaccines are “monovalent,” as they contain only the genetic recipe for the spike protein of the ancestral strain. These monovalent vaccines have been used for the primary vaccination series in the United States.
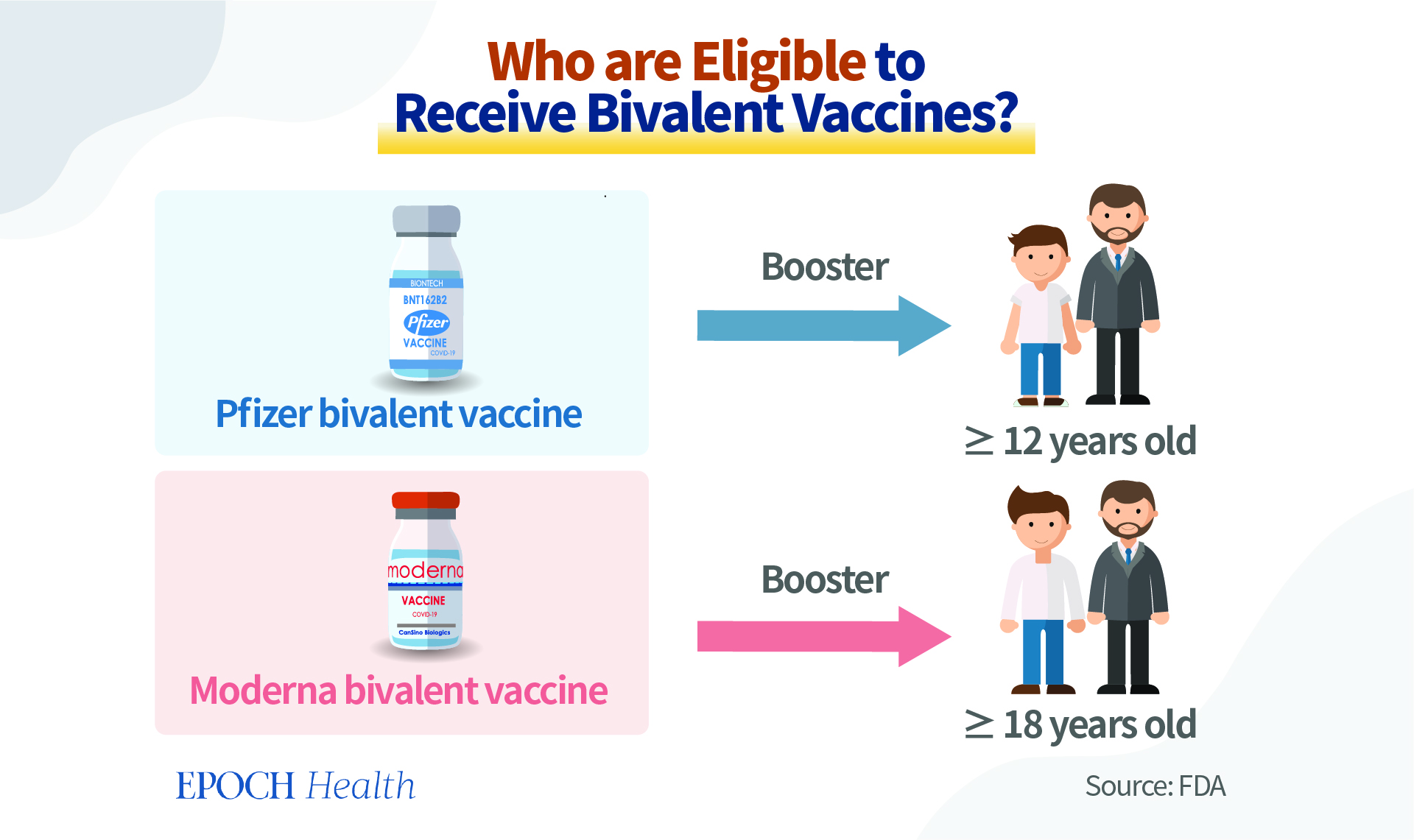
The Pfizer-BioNTech COVID-19 bivalent vaccine is authorized for use as a single booster dose among individuals 12 years of age and older. The Moderna bivalent vaccine is authorized to be used among individuals 18 years of age and older, also as a booster vaccine.
Although theoretically the bivalent vaccines can be used as an initial course of vaccination, two doses will be needed. However, currently, they are considered only a booster, not for the primary vaccination series. For the near future, people initiating a COVID-19 vaccination schedule will still receive the monovalent COVID-19 vaccines for their initial jabs. Individuals are eligible to receive a bivalent COVID-19 booster if they completed their primary vaccination series or received their most recent monovalent COVID booster at least two months ago.
Do Bivalent Vaccines Have Higher Efficacy Than Monovalent Vaccines? Not Likely
The new bivalent vaccines (updated boosters) contain an mRNA component of the ancestral strain, which has already disappeared and been replaced by many variants. One may wonder: why do these new vaccines contain the genetic information of a strain that no longer exists, especially when they were designed to prevent the Omicron variant infection? There are possibly several reasons.
According to the CDC, the mRNA of the original strain is included in order to stimulate an immune response among the vaccine recipients to protect them against the COVID-19 infection in general.
Deepta Bhattacharya, an immunobiology professor at the University of Arizona, believes that the original vaccine recipe is still needed. According to him, it doesn’t make sense to have an Omicron-only vaccine, especially as a primary series, because besides Omicron, there are still the other (previously dominant) variants.
Furthermore, scientists and researchers have always been trying to predict the next variants to emerge. So if the upcoming variants are closely related to the original strain, then the bivalent vaccines would make sense. However, the future is difficult to predict, and nobody can say for sure what the next variants will be like.
According to the general trend of virus development, the COVID-19 virus is constantly breaking through the limitations of its transmission. As it’s becoming increasingly transmissible, its pathogenicity is also decreasing. Therefore, the virus is now becoming more adapted to a long-term presence in human societies. So, it’s unlikely that the virus development will reverse its trend for the next variant to become more close to the ancestral strain than the rest.
Hana El Sahly, a vaccine development expert at Baylor College of Medicine, holds a different opinion from Professor Bhattacharya. According to her, there is no biological reason to include two versions of spike protein from the ancestral strain and the Omicron variant, since the original strain has long gone.
In fact, monovalent vaccines can protect against different COVID-19 strains, although they focus on one particular strain. For instance, the BA.1 monovalent vaccines were manufactured in early 2022 to target the BA.1 sub-lineage. In practice, however, these vaccines can also target the original strain to a certain extent. This is because the virus’s targeted mutations are at the individual protein loci, but the virus itself has many antigenic parts on different proteins that are very similar to the original strain. Therefore, even after receiving the monovalent mutation vaccine, the body will still be able to produce antibodies against the original strain. Similarly, monovalent vaccines focusing on the ancestral strain can also produce antibodies against various mutant strains, to a certain extent.
According to Pfizer, in a study involving participants who received a 30-µg booster dose of the company’s Omicron BA.1 bivalent vaccine, the booster dose produced a superior immune response against Omicron BA.1 subvariant, in comparison with Pfizer’s original COVID-19 vaccine that is monovalent. A 9-fold improvement was shown in the neutralizing titers. Therefore, according to this study, bivalent vaccines are slightly less effective than their monovalent counterparts in terms of antibody neutralizing effects that the vaccines stimulated.
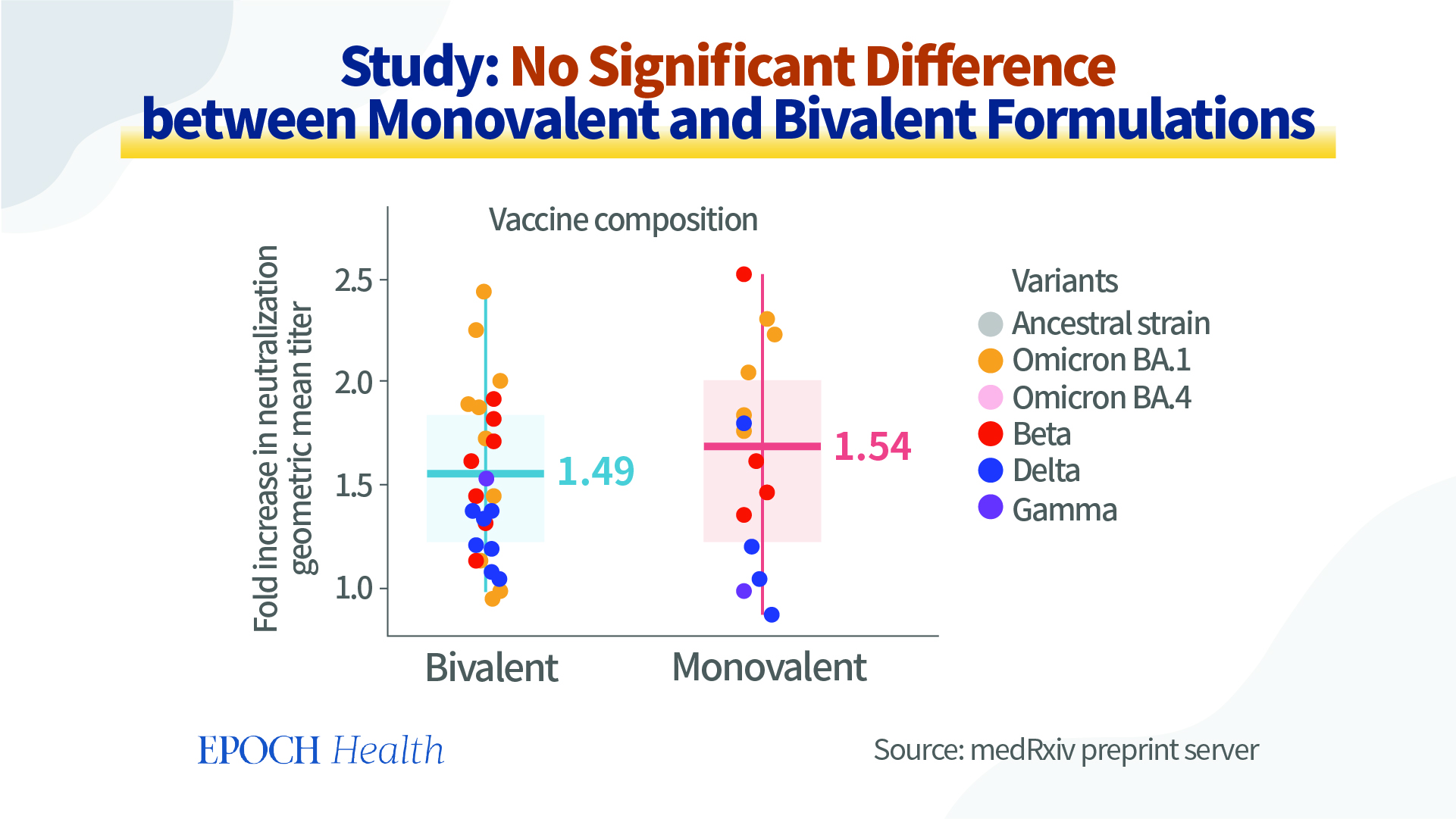
However, according to a preprint study posted in August 2022 on the medRxiv preprint server (pdf), no significant differences between monovalent and bivalent vaccines were discovered. In this study, several researchers from Australia perform a meta-analysis of the data on neutralization titers from clinical studies comparing vaccines based on the original strain and variant-modified vaccines. They’ve conclude that the use of a variant-modified vaccine (i.e. bivalent vaccine) is expected to provide a modest increase in protection, which may be slightly greater in cases where the vaccine immunogen is more antigenically related to the circulating variant or if immunity has waned, but no significant differences were found when stratifying results for monovalent versus bivalent vaccines.
Furthermore, there is no evidence to show that bivalent vaccines are more effective in reducing severe disease. Moreover, depending on the direction of the next coronavirus mutation, the severe disease rate may not increase any more.
Overstock and Wastage of the Original Vaccines: the Real Reason Behind Bivalent Vaccine Development?
When producing the bivalent vaccines, the original-strain based vaccines produced by Pfizer and Moderna can be used.
Immediately after the FDA authorized the use of bivalent vaccines, on September 1, 2022, Health Canada also authorized an Omicron-containing Moderna bivalent vaccine as a booster shot for adults. Moderna and the Canadian government recently agreed to convert 6 million doses of the company’s original COVID-19 vaccines to produce 12 million doses of the bivalent vaccines containing the mRNA of the BA.1 subvariant, despite the fact that the BA.4 and BA.5 subvariants currently represent approximately 94 percent of the virus circulating in Canada, while the BA.1 subvariant represents only 0.1 percent.
The conversion of the original COVID-19 vaccines to the new bivalent booster shots can potentially reduce the stockpile or wastage of these vaccines. Therefore, it makes commercial and financial sense, especially for the vaccine manufacturers and some national governments, who’ve made massive-scale purchases of such vaccines.
According to the Fortune magazine, there is currently a global surplus of original COVID vaccines. As a result, the vaccine makers in many countries are halting production or facing shutdowns as demand for shots wanes. For instance, South Africa is preparing to destroy vaccines that will expire and shut down costly mass-vaccination programs. The Serum Institute, one of India’s major vaccine producers, recently halted the production of its vaccines, as it now has 200 million doses of stockpiled vaccines.
The U.S. government has previously pledged to donate 1.2 billion doses of vaccines, but so far, only less than half have been distributed. According to White House Press Secretary Jen Psaki, the vaccines are becoming more difficult to place, due to the full reserves of other countries. In order to fulfill its pledge, the U.S. government is now sending some pediatric doses, for which the demand is higher, instead of adult Pfizer vaccines. Overall, the demand from other countries is dwindling.
A similar situation is taking place in Canada, as well. Canada’s shipment of vaccines to some low-income countries has been slow and very close to their expiry dates, prompting concerns from experts about the potential vaccine wastage. Ananya Tina Banerjee, assistant professor in McGill University’s epidemiology, biostatistics and occupational health department, believes that the Canadian government is going to waste more vaccines. And according to the Public Health Agency of Canada, as of September 2, 2022, there were more than eight million mRNA vaccines stored in the Canadian federal government facilities.
Therefore, given the large amount of soon-to-be expired original vaccines, converting them into (potentially) in-demand bivalent vaccines can effectively prevent wastage.
In summary, there are relatively large stockpiles of unused monovalent vaccines currently stored in government warehouses, or the government has already paid and placed orders for more such vaccines. These vaccines might have been partially produced and are currently sitting in the vaccine companies’ warehouses. What will happen to these vaccines? Some of the vaccines that were produced too early will also face expiration.
Are the governments implementing bivalent vaccines now to help the vaccine companies or the governments themselves to solve the stockpile problem? The production of these mRNA vaccines has used a lot of resources, and they may feel it would be a shame to waste them in this way. So we can’t rule out the possibility that the authorities will buy bivalent vaccines in order to save resources from being wasted.
So, the ultimate question here is: Is the decision of choosing bivalent vaccine over monovalent vaccine based on commercial or industrial reasons or scientific data?
No Clinical Data Available for Newly Authorized Bivalent Vaccines
Although the FDA has authorized the use of the Pfizer and Moderna bivalent boosters targeting BA.4/5, these vaccines don’t have clinical data. And human data are only available for their bivalent boosters targeting the BA.1 subvariant. According to the companies, clinical trials for the BA.4/5 bivalent vaccines will start in September 2022.
For the BA.4/BA.5 boosters, both companies have submitted only animal data to the FDA, but they haven’t been released publicly. It is only known that at the June 2022 FDA meeting, Pfizer presented their preliminary findings regarding their bivalent vaccine. However, these data are based on only eight mice which were given the vaccines as their third dose. Compared with the original vaccine being used as a booster, the updated boosters have provided the mice with an increased response to all Omicron variants tested, including BA.1, BA.4, and BA.5.
One may wonder: how can the authorities authorize vaccines without human trial data? First, these bivalent vaccines have only been approved for emergency use authorization. In order to obtain the FDA’s full approval, clinical data are a must. Another way is to produce COVID-19 vaccines in the same approach used in the production of influenza vaccines.
Flu vaccines are updated each spring, by predicting the strain that will become prevalent in the fall and winter. Such updated vaccines don’t need new clinical trials to receive approval, unless the manufacturers significantly change the formulations. It can be argued that when producing updated COVID-19 vaccines targeting a specific new variant, the changes to the mRNA used in the vaccines are small, so there’s no need for new clinical trials. Therefore, producing updated vaccines without costly human trials can save both time and money.
Nevertheless, authorizing the use of vaccines without clinical data can lead to lower acceptance by the public, since people won’t be able to know the potential side effects or adverse events until they receive the jab.
Furthermore, the COVID-19 vaccines are different from flu vaccines, as the former have always been experimental, and the latter have been in use for decades, and the public are well aware of the possible side effects of the flu vaccines. Since the COVID-19 vaccines are very new, its side effects/adverse events, especially long-term ones, are still being discovered. Therefore, it’s not an evidence-based evaluation and decision for the authorities or manufacturers to treat COVID-19 vaccines as flu vaccines.
Will the Vaccines Be on the Commercial Market?
On August 11, 2022, the CDC reversed its COVID-19 guidelines. The CDC now recommends people to take personal responsibility to choose their own prevention behaviors, based on their own risk for severe illness and their risk tolerance. Also, as breakthrough infection can take place after vaccination, the COVID-19 prevention recommendations no longer differentiate, regardless of vaccination status. For instance, quarantine of exposed persons is no longer recommended, regardless of their vaccination status.
In the previous months, many people were forced to leave their jobs or stay away from certain places such as universities and schools, since they were unvaccinated. Many people got the COVID-19 jabs in order to go to work, participate in social activities, and travel. However, according to the current CDC recommendations, the vaccination status should no longer be a consideration. In this case, many people will likely choose not to receive the boosters.
So, what will this mean to the vaccine industry, especially the COVID-19 vaccine manufacturers that have made a fortune during the pandemic?
According to the Brookings Institution, the manufacturers’ revenue from selling COVID-19 vaccines in the United States alone, including Pfizer, Moderna, and Johnson & Johnson, totaled $14 billion through the end of 2021.
During the pandemic, the FDA used its EUAs to make many COVID-19 vaccines available to the public, instead of using its regular approval process. Now with new COVID-19 cases in decline and the pandemic seemingly coming to an end, will the federal government create a permanent mandatory program to cover vaccines?
So far, the Biden administration has already ordered 170 million doses of the new bivalent vaccines. And it’s planning to transition to a more standard vaccine purchasing process through different health care system channels, including commercial insurers.
Once on the commercial market, the vaccine and pharmaceutical companies may then raise their products’ prices.
Views expressed in this article are the opinions of the author and do not necessarily reflect the views of NTD Canada.